
Self-Supporting Lattice Telecommunication Towers
October 9, 2024
Nonlinear Performance Analysis of Steel Lattice Energy Distribution Towers
October 23, 2024Study on Corrosion and Mechanical Properties of Steel for Steel Tower
Study on Corrosion and Mechanical Properties of New Steel for Iron Tower
(1 School of Metallurgy and Power Engineering, Chongqing University of Science and Technology, Chongqing 401331;2 Sino-singapore (Chongqing) Ultra High Strength Materials Research Institute Co. , Ltd. , Chongqing 401326;3 Hangta Communication Co. , Ltd. , Anhui Suzhou 234000, China)
Abstract: One Q235B and three Q420B tower steels with different alloy elements were selected for chemicalcompositon analysis by atomic emission spectrometer. NaCl solution was selected as a liquid environment for actionpotential polarization and electrochemical impedance testing of corrosion properties, chemical immersion test to study themorphology and products, mechanical properties were analyzed by tensile test. The results showed that the corrosionperformance of the three Q420B steels was better than that of Q235B, due to the different content of Si, Cr and otherelements, the stability of passivated films on the surface of the materials was different, resulting in different corrosionproperties, the content of element V was increased to optimize the mechanical properties, JMatPro software simulationproved that increasing Mn content could promote tissue stability and improve mechanical properties.

Key words: steel for tower; corrosion performance; polarization curve; mechanical propeties; continuous coolingcurve
Steel used for towers is an important part of the communication system. The safety of communication base station towers is the basic premise for ensuring the normal operation of the communication system, and it is also an important part of the life cycle cost control. As an alternative to Q235 angle steel, Q420B high-strength steel has shown great application potential in engineering structures due to its light weight and high strength. It has obvious advantages in structural safety, energy saving and environmental protection, and can produce good economic benefits. It is widely used in the communication industry3-4.
Communication base station towers are the basis of the communication network. The failure or collapse of a tower usually leads to a domino effect, affecting multiple adjacent towers, which will not only cause huge economic losses, but also cause regional communication networks to be paralyzed, and even social chaos5. Despite the current many design specifications and guidelines, the collapse and damage of towers can still be observed around the world6.
One of the reasons for the collapse and damage of towers is that the service environment is complex and diverse. The surface of the tower material will produce different degrees of corrosion due to the acidity and alkalinity of the environment, resulting in
serious consequences such as rust and loss of materials. According to relevant research, high-strength steel has high strength and bearing capacity. The use of Q420 angle steel can reduce the material weight by 4.9%~7.8%. If Q420B high-strength large-size angle steel replaces Q235B angle steel, it can effectively reduce the overall weight of the tower, save steel, and reduce transportation and installation costs. Therefore, it is of great significance to develop large-size, high-strength and low-temperature impact-resistant angle steel for towers [8.
In order to explore the differences in corrosion and mechanical properties of Q420B relative to Q235B, three Q420B plates with different alloy element contents and one 235B plate were selected within the national standard range. At the same time, the JMatPro software simulation proved that the alloy element content has an important influence on the corrosion and mechanical properties of the material7.

Experimental materials and methods
The two experimental materials were selected from 200mm×500mm×8mm plates, Q235B steel sampling number 1#, Q420B steel sampling number 2#-4#. The samples were cut according to the specifications of 10mm×10mm×8mm, polished with sandpaper, rinsed and dried, and the chemical composition of the samples was determined by atomic emission spectrometer. The results are shown in Table 1.
The samples were cut with the same specifications, polished to 2000 mesh with sandpaper, and surface corrosion was performed with nitric acid alcohol solution after polishing and drying, and microstructure observation was performed by scanning electron microscope. The electrochemical experiment was carried out at room temperature using PARSTAT4000 electrochemical workstation, using a three-pole test system, the reference electrode (RE) used a calomel electrode, the comparison electrode (CE) used a platinum electrode, and the working electrode (WE) was 1#~4# samples. The working solution was 3.5% NaCl solution. During the electrochemical experiment, the open circuit potential test time was 1200s; after the impedance test, the open circuit potential was retested for 600s. The electrochemical drilling impedance spectrum test results were expressed using the Nyquist spectrum.
The immersion test was carried out at room temperature. The samples were selected as 1# sample and 4# sample of Q420B steel (the chemical composition of the two experiments was the largest). Three experimental conditions were set, namely dilute HCl (pH=3), NaOH (pH=11) and 5% NaCl solution. The immersion time was 168h. After the immersion was completed, the samples were taken out, rinsed with anhydrous ethanol and dried. The corrosion morphology was observed by scanning electron microscopy, and the type of corrosion products was explored by surface scanning. The mechanical properties of the material were determined by tensile test. According to the provisions of “Room Temperature Tensile Test Method for Metallic Materials”, a standard specimen was taken from each of the 1#~4# samples. The specific size and shape of the standard specimen are shown in Figure 1, where the width b=30mm and the length b=30mm; the hardness test was carried out by Vickers hardness tester, and 10 measurement points were selected. The results were averaged after removing the extreme values. In this study, the static CCT curve was obtained by simulating different gradient Mn contents through JMatPro software, and the changes in material structure and performance were further analyzed.
2 Experimental results
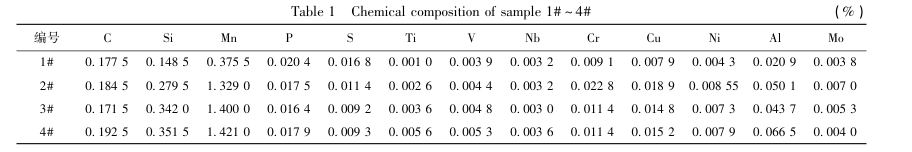
The SEM microstructure of the 1#~4# samples in the original state is shown in Figure 2(a). The 1# sample has irregular structure and unclear structure characteristics. The 2#~4# samples are pearlite. In addition to the clear rolling direction of the microstructure, the matrix pearlite structure is layered.
Figure 2 (b~c) shows the polarization curves and Nyquist spectra of the 1#~4# samples obtained by the electrochemical workstation under the conditions of 3.5% NaCl neutral solution. The corrosion potential (E) and corrosion current density (J) calculated from the polarization curves are shown in Table 2. In the neutral solution environment, the corrosion potential (E_corr) of the 1# sample is -0.863V, and the corrosion potentials of the 2#~4# samples are -0.871V, -0.737V and -0.710V, respectively, which tend to be positive overall. During the electrochemical experiment, the anode regions of the four samples all showed varying degrees of passivation, resulting in varying degrees of fluctuation in the polarization curves, as shown in Figure 2(b). Figure 2(c) shows the electrochemical impedance spectra of samples 1#~4#, where the impedance radius of sample 1# is incomplete, while the impedance of sample 4# is the most complete. In neutral solution, the corrosion resistance of the two samples is quite different. On the contrary, the impedance radius of samples 2#~3# is relatively small, and their corrosion resistance is similar in a neutral solution environment.
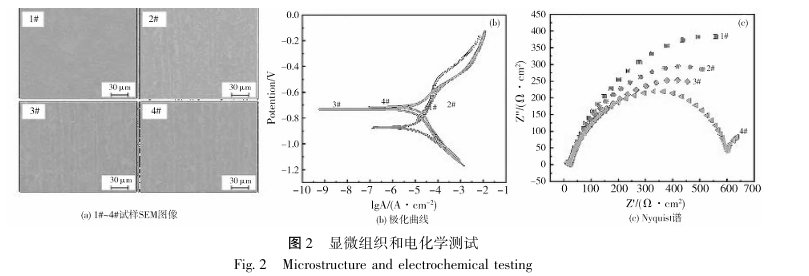
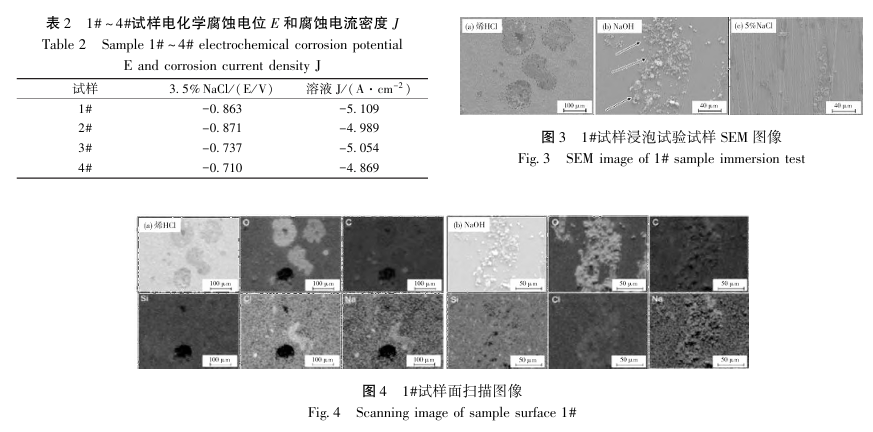
After the 1# sample was immersed in three different solutions of dilute HCl, NaOH and 5% NaCl, its SEM image is shown in Figure 3. As can be seen from Figure 3a, under acidic conditions, pitting corrosion occurs, and there are corrosion pits of varying degrees and numbers on the surface of the sample. The chemical composition of the corrosion area is shown in Figure 4a, and the corrosion area mainly shows the enrichment of O. After the sample was immersed in an alkaline solution, regional, dense, and different-shaped pearlescent particles appeared on the surface of the sample, as shown in Figure 3b; the chemical composition of the particles is shown in Figure 4b, and only the signal of the O element is the strongest. Therefore, in an alkaline environment, serious oxidation occurs on the surface of the sample. The corrosion in an alkaline environment mainly reduces the service life of the material through oxidation. After being immersed in a 5% NaCl solution, the surface structure of the sample did not undergo obvious corrosion, and the corrosion performance of the material was relatively stable in a neutral liquid environment.
After the 4# sample was immersed in three different solutions, namely, dilute HCl, NaOH and 5% NaCl solution, its SEM image is shown in Figure 5. In Figure 5a, it can be observed that there are no corrosion pits and corrosion particles in the corrosion area on the surface of the sample, and the corrosion area is small with few corrosion points. The chemical composition of the corrosion area is shown in Figure 6a. There is an obvious O enrichment phenomenon inside the corrosion area, but there is no enrichment of elements such as C, Si, Cl and Na. After the sample was immersed in an alkaline solution, pearlescent particles were attached (Figure 5b); the chemical composition of the corrosion product is determined as shown in Figure 6b. O is enriched in the corrosion area on the surface of the sample, and the material oxidation phenomenon is serious under alkaline solution conditions.
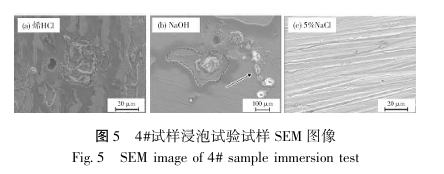

Fig. 7 Stress-strain curve and continuous cooling curve
Fig. 7(a) is the stress-strain curve of steel along the rolling direction. The horizontal axis is the nominal strain of the specimen within the extensometer gauge length of 50mm; the vertical axis is the average stress of the middle cross section of the specimen. The stress-strain curve includes elastic stage, yield stage, strengthening stage and failure stage. The elastic stage stress of the 1# specimen is significantly lower than that of the other three specimens. Its tensile strength and yield strength are 458.9MPa and 328MPa respectively, and the elongation is only 27.5%. The tensile strength and yield strength of the 2#~4# specimens are both increasing, with the maximum values reaching 555MPa and 379.3MPa respectively. The elongation distribution range is 25.8%~31.0%, and the average elongation is 28.4%, which is greater than the elongation of the 1# specimen. Comprehensively considering the three mechanical properties, the 1# specimen has the worst mechanical properties, and the 4# specimen has the best mechanical properties.
The simulation results of the CCT curve corresponding to different Mn contents are shown in Fig. 7(b). Among them, F represents ferrite, P represents pearlite, and M represents martensite transformation temperature; A_c represents the starting temperature of the transformation from heating process to austenite; A_c3 represents the end temperature of the structural change of steel in the heating process stage to completely transform into austenite. Figures a and b are the continuous cooling (CCT) curves of samples 1# and 4#, respectively. During the continuous cooling process at different cooling rates, the supercooled austenite will undergo different phase transformations, and the formed structure and morphology will change accordingly.
According to the measurement, the austenite start transformation temperature A_c1 point during the heating of the original sample is 722.3℃, and the transformation end temperature A_c3 point is 849.5℃. With the increase of cooling rate, the starting point of phase transformation shows a downward trend, and the microstructure gradually transitions from ferrite and pearlite to bainite. When the cooling rate is less than 1℃/s, the precipitation of pearlite gradually increases, and bainite will appear if the cooling is continued. When the cooling rate reaches 1~10^∘ C/s, the precipitation of pearlite reaches its maximum. With the increase of cooling rate, when it exceeds 10℃/s, the precipitation phase is mainly bainite. If the cooling is continued, the supercooled austenite will eventually turn into martensite. When the Mn content increases, the austenite transformation temperature during heating becomes 705.9℃, and the transformation end temperature becomes 822.4℃. The minimum rate of pearlite maximum precipitation is greater than 1℃/s, and the martensite transformation point M decreases.
3 Analysis and discussion
The essence of pearlite is a phase mixture of ferrite and cementite. Sample 1# Q235B is a material with ferrite as the main component. In the C-rich area, due to deformation treatment such as forging, stretching or extrusion, the C-rich micro-area is enlarged, the C content concentration is reduced, and the continuous cementite is difficult to precipitate in equilibrium. It is only distributed on the surface of the matrix in a dotted manner, showing an uneven pearlite structure.
The corrosion potential of sample 1# in NaCl solution is -0.863V, and the corrosion current density (J_corr) is -5.109A·cm^(-2). The corrosion potential of sample 4# in NaCl solution is -0.710V, and the corrosion current density is -4.869A·cm^(-2). In general, the smaller the corrosion current density, the more positive the corrosion potential, the larger the impedance radius, and the better the corrosion performance of the material12. Its corrosion current density is generally used to evaluate the corrosion rate. It can be seen that oxide films such as Cr_2 O_3 effectively slow down the corrosion rate. Since the liquid environment is rich in CI, the material mainly undergoes oxygen absorption corrosion, and the Fe in the matrix is oxidized into Fe oxides, among which B-FeOOH is easily formed. Its tunnel structure in the unit cell allows CI in the solution to further diffuse, resulting in the destruction of the stability of the passive film on the surface of the material. Since it is very easy to adhere to the surface of the material matrix, it has an inhibitory effect on the formation of the passive film. In Q235B, the content of important alloying elements such as Si, Mn, and Cr is relatively low, and the oxide film is not easy to exist, resulting in poor corrosion performance of the material. After immersion, the corrosion morphology of the two materials in different solutions is significantly different. There is no macroscopic change on the surface of the two materials in 5% NaCl solution, but after immersion in acidic and alkaline solutions, the surface corrosion degree of 1# material is significantly greater than that of 4# Q420B material. Due to the high concentration of CI ions and low Cr content, the stability of the passive film is poor. Under acidic conditions, corrosion pits appear on the surface of the substrate of material 1#, and the degree of corrosion is greater than that of material 4#, showing a pitting corrosion phenomenon; under alkaline conditions, different numbers of particles appear on the surface of the two materials. After surface scanning, it can be determined that the particles are mainly enriched with oxides, and oxidation corrosion reduces the corrosion performance of the material. The degree of adhesion of oxide particles in sample 1# is much greater than that of sample 4#, and the corrosion resistance is poor.
In steel materials, the role of V is similar to that of Cr. It combines with carbon elements to form carbides, which has the effect of hindering graphitization. The increase in V content can effectively improve the hardness and tensile properties of the material. As the content of alloying elements such as Mn in the material gradually increases, it gradually exhibits excellent mechanical properties such as elongation and yield strength during the tensile process15. The Mn element will be distributed from ferrite to austenite, making the local austenite gradually rich in Mn. These Mn-rich austenites will prevent the migration of ferrite grain boundaries, further inhibit the growth and coarsening of grains, and improve the stability of Mn-rich austenite; during the heating process, the increase in Mn content increases the nucleation position of ferrite, refines the structure, increases the stability of ferrite, and effectively reduces the transformation temperature from ferrite to austenite7; the alloying element Mn produces Al_6 after solution annealing Mn particles are dispersed in the matrix, improving the hardness of the material. When its content increases, the M point that promotes martensitic transformation is reduced, the organization is improved, and the mechanical properties of the material are improved. 18
4 Conclusion
(1) The electrochemical corrosion performance of Q235B steel is poorer than that of Q420B steel. Under acidic conditions, pitting corrosion occurs on the surface of Q235B steel, and the corrosion degree is greater than that of Q420B steel. Under alkaline conditions, the oxidation degree is deep, and the oxide particles of Q235B steel are attached to the matrix. Under 5% NaCl conditions, no macroscopic corrosion occurs in the two materials. Due to the presence of Si, Cr, etc. in Q420B steel, the corrosion rate is relatively high. The high content of alloying elements improves the stability of the passive film on the surface of the material, reduces the adhesion and intrusion of CI and the oxidation rate of the material, and exhibits excellent corrosion resistance in the simulated application environment;
(2) The mechanical properties of Q420B steel are better than those of Q235B steel. The increase of alloying element V effectively inhibits graphitization and improves the hardness and tensile properties of the material;
(3) After the simulated alloying element Mn content is increased, the ferrite grains are refined and the organizational stability is improved; its dispersed particles strengthen the matrix, reduce the martensitic phase transformation temperature, improve the organization, and improve the mechanical properties.










